Questionnaire
This is a preview of the questions you will be asked if you sign up. When you are filling in the real questionnaire online, you will be able to input your answers by selecting the tick buttons, or by entering your details in the text boxes.
Data sharing
Additional consent
We would like to ask for your consent to allow the following secure exchange of your data. If you are happy to do so, please select ‘Yes’ below each statement. If you choose not to give consent to these statements, your participation in the patient registry remains unaffected and you are still most welcome.
SMA REACH UK is a research study that collects longitudinal clinical data from children with SMA. It is operated by Great Ormond Street Hospital and the MRC Neuromuscular Centres in London and Newcastle (John Walton Muscular Dystrophy Research Centre, JWMDRC). Adult SMA REACH is a research study that collects longitudinal clinical data from adult SMA patients. It is coordinated from the JWMDRC, Newcastle University. Multiple neuromuscular centres across the UK participate in these studies. Further detail is included within the Participant Information and Consent statements.
Note: If you are not yet participating in one of the below studies but are willing to share your registry data should you participate in them in the future, please select ‘Yes’.
I give my permission for my data to be securely exchanged with SMA REACH UK (children) or with Adult SMA REACH (if I have consented to participate in one of these studies).
I give my permission for my anonymous PROMs data to be used to inform regulatory medical authorities in the UK (if I have consented to participate in SMA REACH UK or Adult SMA REACH).
I give my permission for the anonymous linkage of my information in the UK SMA Patient Registry with the Newcastle MRC Centre Biobank for Rare and Neuromuscular Diseases (if I have consented to participate in the Biobank study).
SMA REACH reference number
If you are participating in SMA REACH UK or Adult SMA REACH you will have been given an SMA REACH reference number. If easily to hand, please add the reference number below. This will assist the linking of patient registry and SMA REACH data and minimise the sharing of identifiable data.
Personal data
What is your NHS or PPS number?
The NHS number is a patient’s unique ID number in the National Health System - everybody in the United Kingdom has one. You will find it on your medical card. If you can't find it, you can ask your GP.
What were your name and sex at birth?
Please enter this data even if your data has not changed since birth.
Where were you born?
Diagnosis
What is your diagnosis, according to your doctor?
For more information on the types of SMA, please see the guide to the standards of care (link opens in new window).
When did your SMA symptoms begin?
When did you receive your SMA diagnosis?
Quality of life
Under each heading, please tick the one box that best describes your health today.
(e.g. work, study, housework, family or leisure activities)
- We would like to know how good or bad your health is today.
- This scale is numbered from 0 to 100.
- 100 means the best health you can imagine.
0 means the worst health you can imagine. - Please indicate on the scale how your health is today.
Overall condition
How are you currently affected by your SMA?
How do you feel your overall condition has changed from 6 months ago?
Reproduced from Guy W (ed). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education and Welfare Public Health Service Alcohol, Drug Abuse and Mental Health Administration, 1976
Further comments
We understand that it can sometimes be difficult to describe your experiences of life with SMA using a measurement scale. Therefore, if you are able and would like, please add a comment in your own words to describe your experiences over the last few months of living with SMA, for example your general health (physical and/or mental), your experience of SMA treatments and your quality of life. Please try to limit your response to about five sentences.
Genetic report
Knowing the precise details of an individual's gene mutation will add to our understanding of spinal muscular atrophy. In addition, a confirmed genetic mutation is important when considering possible SMA therapy options with your doctor.
If you have the genetic report yourself (or any other document that includes details of the genetic diagnosis) please click on the following link to upload the document:
Upload document (link opens in new window)
Please also enter the name and contact details of the hospital, medical centre or geneticist centre where the genetic test was performed in the field below. In case anything is missing, we can obtain the correct document for you from them.
If you do not have the genetic report yourself (or the results of the genetic test are pending), please enter the name and contact details of the hospital, medical centre or geneticist where the test was performed in the field below. We will then contact them and ask for a copy of the report.
If a genetic test has not been performed yet, please contact your doctor, as knowing the details of the mutation is very important for an appropriate treatment.
Status of the genetic report
If you have the genetic report yourself, please upload it to the upload page (link opens in new window).
Family
Do you know of anybody else in your family who has SMA or similar symptoms?
Since SMA is an inherited condition, it is important for us to know if there are any relatives who have similar symptoms or the same diagnosis.
If yes, which family member(s) are affected?
To add more than one family member, click on the button “Add another family member” below.
For example, if the affected relative is an uncle, select “Male” if he is your father’s brother and “Female” if he is your mother’s brother. In the case of an affected grandchild, please select “Male” if the grandchild is a son’s child, and “Female” if it is a daughter’s child. In the case of a niece or nephew, select “Male” if the affected relative is your brother’s child and “Female” for your sister’s child.
For parents, full siblings, or children, leave this option unspecified.
Contractures
Do you have contractures in any of the areas listed below?
A contracture is a decreased range of motion around a joint.
Scoliosis
Do you suffer from scoliosis (curvature of the spine)?
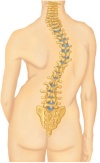 Some SMA patients suffer from weakness in their back muscles which results in a deformation or “bending” of their spine called scoliosis.
Some SMA patients suffer from weakness in their back muscles which results in a deformation or “bending” of their spine called scoliosis.
Have you had spinal surgery for scoliosis?
Basic motor function
Are or were you able to hold your head up without support?
If applicable:
I gained this ability at the age of years and months.Are or were you able to roll onto your side?
If applicable:
I gained this ability at the age of years and months.Are or were you able to sit without support?
If applicable:
I gained this ability at the age of years and months.Are or were you able to crawl on hands and knees?
If applicable:
I gained this ability at the age of years and months.Are or were you able to stand with assistance?
If applicable:
I gained this ability at the age of years and months.Are or were you able to stand alone (without assistance)?
If applicable:
I gained this ability at the age of years and months.Advanced motor function
Are or were you able to walk with assistance?
If applicable:
I gained this ability at the age of years and months.Are or were you able to walk alone (without assistance)?
If applicable:
I gained this ability at the age of years and months.Are or were you able to walk 10 metres alone (without assistance)?
If applicable:
I gained this ability at the age of years and months.Are or were you able to climb stairs?
If applicable:
I gained this ability at the age of years and months.Are or were you able to achieve useful function of hands (e.g. hold a pen or pick pennies up from a table)?
If applicable:
I gained this ability at the age of years and months.Are or were you able to move both arms from your sides to reach overhead (in a sitting position)?
If applicable:
I gained this ability at the age of years and months.Are or were you able to raise your hands to your mouth (in a sitting position)?
If applicable:
I gained this ability at the age of years and months.Wheelchair use
Do you currently use a wheelchair due to your SMA?
Please also include the usage of similar devices such as a mobility scooter or a stroller. However, please do not consider devices that assist you in walking (such as a walker or a cane), or do not support mobility (such as a static standing frame).
If applicable:
I sometimes use a wheelchair for long distances or outside since the age of years and months.Feeding
Do you currently use a gastric or nasal tube for feeding?
SMA patients sometimes have trouble eating and therefore have to be fed through a feeding tube. A gastric tube is one that goes directly into the stomach through an incision in the tummy. A nasal tube (also called nasogastric tube) is one that goes through the nose and down into the stomach.
Have you previously used a gastric or nasal tube for feeding?
If yes, please enter the type of usage and period below. To add a further usage, click on the button “Add further feeding tube usage”.
Breathing
Do you currently use non-invasive ventilation?
Some SMA patients have trouble with their breathing. To support their breathing, they get a ventilation device that they either use full-time, i.e. 24 hours a day, or only for several hours a day or at night. “Non-invasive” means that they use this device without having had an operation. Usually this means they wear a mask that can be removed at any time.
Have you previously used non-invasive ventilation?
If yes, please enter the type of usage and period below. To add a further usage, click on the button “Add further non-invasive ventilation usage”.
Do you currently use invasive ventilation?
“Invasive ventilation” means that the patient had to have an operation (an incision in the wind-pipe, also known as tracheotomy) to use the ventilation device. Again, this ventilatory support system can be used either all day or a few hours per day.
Have you previously used invasive ventilation?
If yes, please enter the type of usage and period below. To add a further usage, click on the button “Add further invasive ventilation usage”.
Do you use any assistance with airway clearance and/or secretion mobilisation?
If yes, please select the type of assistance and frequency of use below.
Has your pulmonary (breathing) function ever been tested?
To monitor your breathing function, a pulmonary function test may have been conducted. One of the parameters measured in these tests is known as the forced vital capacity (FVC). To test the FVC, the patient has to breathe in as far as he or she can and then blow out into a machine that measures how much air is being exhaled.
If yes, what was the result of your most recent pulmonary function test?
The forced vital capacity (FVC) is the volume of air exhaled during a pulmonary function test. On the test report, it may be specified in litres and/or as a percentage. The FVC varies with age, gender, weight and height; therefore, it is compared to the volume which one would expect from a corresponding healthy person, resulting in the percentage. In case you don't know any of the following details, just leave the respective fields blank.
If you had already entered a test result, click on the button “Add further test result” to add another one.
The peak cough flow is the maximum air flow generated during a cough. It is used to assess cough in patients with respiratory muscle weakness and is done using a flowmeter graduated in litres/minute.
litres/minuteSleep and fatigue
Have you ever participated in a sleep study?
A sleep study is a non-invasive, overnight observation that allows doctors to monitor your breathing while you sleep.
Do you experience fatigue?
Medication and therapy
Have you ever taken any medication to specifically manage your SMA?
Such a therapy is also called a disease-modifying therapy. Currently, three such drugs are approved: Spinraza (also called nusinersen), Zolgensma and Evrysdi (risdiplam).
If yes, what medication have you taken?
You can leave any of the following fields blank if you don't know or are unsure. If you have taken more than one medication, click on the button “Add another medication” to add further details.
Has an anti-AAV9 antibody test been performed?
If yes, what were the results?
In case more than one test was performed, click on the button "Add further antibody test" to add further input fields.
Have you taken any other prescribed drugs in the last 12 months?
This may include drugs or supplements for your bone health, your gastrointestinal system, your respiratory system, immunisations such as a flu shot, or other supplements.
Please note that the inclusion of a drug or supplement in this list does not indicate a recommendation.
Which of the following therapies have you received in last 12 months?
Select all that apply.
Hospitalisation and other illnesses
Have you had an overnight stay in a hospital in the past 12 months?
If yes, please provide details for each stay.
To add a further entry, click on the button “Add another hospitalisation”.
Have you been diagnosed with any other illnesses or conditions not related to your SMA in the past 12 months?
In addition to illnesses, pregnancy may also be listed here. If a stay in hospital was necessary due to an illness and you have entered it above, you do not have to enter it again here.
If yes, please provide details for each illness.
To add a further entry, click on the button “Add another illness”.
Clinical trials and other registries
Are you currently participating in a clinical trial or have you participated in one previously?
A clinical trial is a rigorously controlled test designed to examine the safety and/or effectiveness of medicines, devices, treatments, or preventive measures in humans.
If yes, which trial(s) are you participating in or have you participated in?
To add multiple trials, click on the button “Add another clinical trial”.
Are you taking part (or have you previously taken part) in any other SMA-related registries or research studies?
Since we aim to harmonise international SMA registries and include data in one global registry, we want to make sure that patients do not appear in the global registry twice by mistake. If you are already registered in another SMA registry, we will check if that is a problem and contact you if necessary. In any case, please continue your registration here. You can select more than one registry if necessary.
Further details
What are your current height and weight?
Contacting you
Would you like to be contacted in case you might be suitable for a clinical trial?
Please note that even if the coordinators of a clinical trial believe that you might be eligible for the trial, based on the data about you stored in the UK SMA Patient Registry, it is still possible that later on it will turn out that you do not meet the trial inclusion criteria after all. Please also be aware that if we inform you about the existence of a trial, this does not imply that we endorse it. In order to participate in any trial, you will need to fill out a separate informed consent form.
